- Athanassios Antonopoulos
- Review
Out-of-hospital cardiac arrest in a mitral valve prolapse young male patient with acute mitral regurgitation due to cordal rupture. A case with a literature review
- 3/2017-Ottobre
- ISSN 2532-1285
- https://doi.org/10.23832/ITJEM.2017.017

Introduction
Case Report
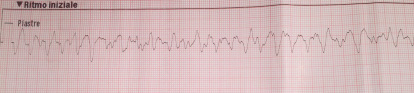
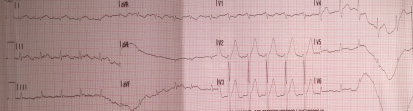
Cardiopulmonary resuscitation was immediately started and continued for 15 minutes (three DC defibrillation 200 Joules for ventricular fibrillation, one synchronized cardioversion 150 Joules for ventricular tachycardia with pulse and a short period of pulseless electrical activity treated with cardiac massage and epinephrine) before returning in spontaneous circulation with sinus tachycardia. During that time, attempts for endotracheal intubation failed, despite continuous suction applied, due a lot of regurgitated food. The patient in coma state (Glasgow coma scale 4) was treated with intravenous sedation to support ventilation with supraglottic tube. The patient was then transported by ambulance to the emergency department of the nearest available hospital for further assistance. Endotracheal intubation was finally obtained and completed with fiberoptic bronchoscopy toilette. Chest x-ray showed ab ingestis pneumonia. Body temperature was normal. Initial blood investigations were as follow: pH 7.026, PCO2 82.9 mmHg, PO2 73.9 mmHg, BEb -11.8 mmol/L, lactates 2.18 mmol/L, potassium 3.62 mmmol/L, sodium 138 mmol/L, hemoglobin (Hb) 16 g/dL, glucose 320 mg/dL, creatinine 1.32 mg/dL, high sensitivity troponin 192 ng/L. Important mitral regurgitation, probably due to ruptured mitral chordae tendinae, was observed by trans-thoracic echocardiography in the emergency room when the patient arrived. Systolic blood pressure was 60 mmHg and infusion of norepinephrine has been started to increase mean pressure. No changes in repolarization were noticed in the first 12 leads ECG after resuscitation (figure 2) but premature ventricular complexes were observed.
Follow-up
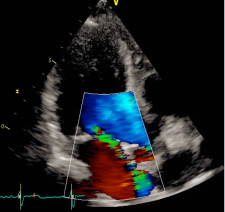
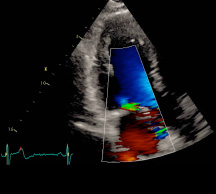
Figure 3: Mitral insufficiency six months after mitral valve repair
Discussion
Till now, the presence of ventricular arrhythmias among patients with MVP has been underappreciated. Some previous studies described an increased incidence of serious ventricular arrhythmias in this kind of patients. On the contrary, other studies demonstrated that occurrence of MVP, among sudden cardiac arrest causes in the community, may not be significantly increased from what is expected in the general population (2, 6). This rare case regards a young male with asymptomatic MVP who was resuscitated from out-of-hospital sudden cardiac arrest. The exact mechanism linking MVP to sudden cardiac arrest is still not known. An autoptic study in patients with MVP had shown that mitral valve leaflet length and posterior leaflet thickness were greater in hearts of patients who died suddenly compared to patients with MVP who died from other causes (7).
Until now the debate continues as to whether MVP is the cause of SCD or merely an association. However, several possibilities have been advanced. Previous studies supposed the hypothesis of an abnormal autonomic and hyperadrenergic state (8). Boudoulas et al (9) demonstrated that patients with MVP had frequent, transient and inappropriate increases of catecholamines during the day or stressful activities. The above condition may produce transient QT prolongation relative to the QS2 interval (electromechanical systole). To further support this hypothesis another study showed that 24-hour catecholamines excretion and frequency of premature ventricular beats were parallel (10). Wilde and colleagues (11) performed detailed mapping studies on a patient with MVP and ventricular tachycardia and concluded that delayed afterdepolarization-induced triggered activity was the mechanism, with stretch and fibrosis of the papillary muscles, contributing to the origin of the arrhythmia.
Furthermore, some studies have suggested that ventricular arrhythmias may be more frequent in MVP when mitral regurgitation is present (12,13,14). This could reflect hemodynamic consequences of volume overload; however it is noteworthy that the mean left ventricular ejection fraction was normal and left ventricular diameter not significantly increased in the MVP patients compared to the cases without MVP in our population. Additionally, it has been proposed that a subset of young patients with isolated MVP and SCD could actually have less mitral regurgitation (15,16). Certain studies have found women to be at higher risk (4, 14) that not regards our patient. An other study, using signal-averaged ECG, identified an increased frequency of late potentials in MVP patients (17); however the utility of this tool in risk prediction is unclear.
The arrhythmic substrate in this setting of patients was also recently investigated by Basso and colleagues (18), who found that in patients who experienced sudden cardiac death with MVP was present fibrosis in the papillary muscles of adjacent free wall and infero-basal wall, as demonstrated by late gadolinium-enhancement on magnetic resonance study. The above features closely overlapping with the histopathological findings which described that kind of fibrosis pattern different in terms of type (i.e. scarring) and location (i.e. papillary muscles and basal potero-lateral wall). Moreover, the degree of MVP and leaflets thickness was found to be related with QT dispersion, providing also a mechanical correlation for electrical disturbances in this subset of patients (19). In a similar way, Sriram et al studying patients with out-of-hospital cardiac arrest in bileaflet mitral valve prolapsed syndrome, found on the electrophysiological study that the earliest site of origin of premature ventricular complexes was mapped to the tips, body or bases of either the anterior or posterior papillary muscles (20). Additionally, it is worth to underline that human mitral valve has distinct patterns of innervation. Mechanical stimuli produced by irregular coaptation of prolapsed mitral cups may cause an abnormal autonomic nerve feedback between the central nervous system and the mitral valve (21).
In our case we did not have the possibility of MRI investigation due to the emergency situation and the electrocardiographic evaluation after resuscitation was in sinus rhythm without significant repolarization changes. Moreover, the explanation of the genesis of ventricular fibrillation in our patient is not clear. We hypothesed that a mechanical stimulation, with an important papillary stretch and the sudden rupture of cordae tendinee, could cause an abnormal nerve feedback between central nervous system and mitral cusps. This is of relevance in most of the previous studies demonstrating the importance of papillary muscle-annular continuity in the maintenance of normal ventricular function after surgical valve correction (12). Consequently, an urgent surgical correction of the above described mechanism as first line therapeutic option, may treat also the arrhythmogenic substrate.
References
- Delling FN, Rong J, Larson MG, Lehman B, Osypiuk E, Stantchev P, Slaugenhaupt SA, Benjamin EJ, Levine RA, Vasan RS. Familial clustering of mitral valve prolapse in the community. Circulation. 2015 Jan 20;131:263-8
-
Devereux RB, Kramer-Fox R, Brown WT, Shear MK, Hartman N, Kligfield P, Lutas EM, Spitzer MC, Litwin SD. Relation between clinical features of the mitral prolapse syndrome and echocardiographically documented mitral valve prolapse. J Am Coll Cardiol. 1986;8:763–72
-
Boudoulas H, Wooley CF (eds): Mitral Valve: Floppy Mitral Valve, Mitral Valve Prolapse, Mitral Valvular Regurgitation, ed 2, revised. Armonk, Futura, 2000
-
Zuppiroli A, Mori F, Favilli S, Barchielli A, Corti G, Montereggi A, et al. Arrhythmias in mitral valve prolapse: relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am Heart J 1994;128:919–927
-
Anders S, Said S, Schulz F, Puschel K. Mitral valve prolapsed syndrome as cause of sudden death in young adults.Forensic Sci Int. 2007;171:127-30
-
Narayanan K, Uy- Evanado A, Teodorescu C, Reinier K, Nichols GA, Gunson K et al. Mitral Valve Prolapse and Sudden Cardiac Arrest in the Community. Heart Rhythm 2016;13:498-503
-
Davies MJ, Moore BP, Braimbridge MV. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J 1978;40:468-81
-
Boudoulas K.D, Boudoulas H. Floppy Mitral Valve (FMV)/Mitral Valve Prolapse (MVP) and the FMV/MVP Syndrome: Pathophysiologic Mechanisms and Pathogenesis of Symptoms. Cardiology 2013;126:69–80
-
Boudoulas H, Reynolds JC, Mazzaferri E, Wooley CF. Mitral Valve Prolapse Syndrome: The Effect of Adrenergic Stimulation. J Am Coll Cardiol 1983;2:638-644
-
Boudoulas H, Reynolds JC, Mazzaferri E, Wooley CF. Metabolic studies in mitral valve prolapse syndrome. A neuroendocrine–cardiovascular process. Circulation 1980;61:1200-1205
-
Wilde AA, Duren DR, Hauer RN, deBakker JM, Bakker PF, Becker AE, Janse MJ. Mitral Valve Prolapse and Ventricular Arrhythmias: Observations in a Patient with a 20-year History. J Cardiovasc Electrophysiol.1997;8:307-16
-
Grigioni F, Enriquez-Sarano M, Ling LH, Bailey KR, Seward JB, Tajik AJ et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol 1999;34: 2078–2085
-
Turker Y, Ozaydin M, Acar G, Ozgul M, Hoscan Y, Varol E, Dogan A, Erdogan D, Yucel H. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int J Cardiovasc Imaging. 2010;26:139–45
-
Kligfield P, Hochreiter C, Kramer H, Devereux RB, Niles N, Kramer-Fox R, Borer JS. Complex arrhythmias in mitral regurgitation with and without mitral valve prolapse: contrast to arrhythmias in mitral valve prolapse without mitral regurgitation. Am J Cardiol. 1985;55:1545–9
-
Dollar AL, Roberts WC. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. J Am Coll Cardiol. 1991;17:921–31
-
La Vecchia L, Ometto R, Centofante P, Varotto L, Bonanno C, Bozzola L, Bevilacqua P, Vincenzi M. Arrhythmic profile, ventricular function, and histomorphometric findings in patients with idiopathic ventricular tachycardia and mitral valve prolapse: clinical and prognostic evaluation. Clin Cardiol. 1998;21:731–5
-
Jabi H, Burger AJ, Orawiec B, Touchon RC. Late potentials in mitral valve prolapse. Am Heart J. 1991;122:1340–5
-
Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A et al. Arrhythmic mitral valve prolapsed and sudden cardiac death. Circulation 2015:132:556-66
-
Zouridakis EG, Parthenakis FI, Kochiadakis GE, Kanoupakis EM, Vardas PE. QT dispersion in patients with mitral valve prolapse is related to the echocardiographic degree of the prolapse and mitral leaflet thickness. Europace 2001;3:292–8
-
Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, et al. Malignant Bileaflet Mitral Valve Prolapsed Syndrome in Patients With Otherwise Idiopathic Out-of-Hospital Cardiac Arrest. J Am Coll Cardiol 2013;62:222-30
-
Boudoulas H, Schaal SI, Wooley CF. Floppy mitral valve/mitral valve prolapse: cardiac arrhythmias; Pacing and Electrophysiology. Dordrecht: Kluwer Academic;1998:pp 89-95