- Claudia Cicchini
- Original Article
Measures of efficiency in the emergency department observation units
- 3/2018-Ottobre
- ISSN 2532-1285
- https://doi.org/10.23832/ITJEM.2018.035

Abstract
Key words: observation unit, emergency department, emergency medicine, efficiency
Financial aspects of the EDOU USE
|
Triage color code
|
Rate per patient (euro)
|
|
Dead on arrival
|
25.82
|
|
Red
|
1032.91
|
|
Yellow
|
309.87
|
|
Green
|
154.94
|
|
White
|
41.32
|
Measures of observation care
Conclusions
References
- Dickfos M, Ibrahim H, Evans A, Franza R. Cohort study on emergency general surgery patients and an observation unit. S Afr J Surg 2017; 55(3): 66.
- Peacock F, Beckley P, Clark C, et al. Recommendations for the evaluation and management of observation services: a consensus white paper: the Society of Cardiovascular Patient Care.Crit Pathw Cardiol. 2014 Dec;13(4):163-98
- Pena ME, Kazan VM, Helmreich MN, Mace SE.Care of Respiratory Conditions in an Observation Unit.Emerg Med Clin North Am. 2017 Aug;35(3):625-645
- Stewart M, Bledsoe J, Madsen T, et al.Utilization and Safety of a Pulmonary Embolism Treatment Protocol in an Emergency Department Observation Unit.Crit Pathw Cardiol. 2015 Sep;14(3):87-9
- Mong R, Arciaga GJ, Tan HH. Use of a 23-hour emergency department observation unit for the management of patients with toxic exposures.Emerg Med J. 2017 Nov;34(11):755-760
- Wheatley MA.Additional Conditions Amenable to Observation Care.Emerg Med Clin North Am. 2017 Aug;35(3):701-712
- Wheatley MA, Ross MA.Care of Neurologic Conditions in an Observation Unit.Emerg Med Clin North Am. 2017 Aug;35(3):603-623
- Ham JJ, Ordonez E, Wilkerson RG. Care of Acute Gastrointestinal Conditions in the Observation Unit.Emerg Med Clin North Am. 2017 Aug;35(3):571-587
- Abetz JW, Adams NG, Mitra B. Skin and soft tissue infection management failure in the emergency department observation unit: a systematic review.Emerg Med J. 2018 Jan;35(1):56-61
- Simone A, Livoli D, Valeriano V, Pugliese F.R. Short Term Observation: 2008-2015 Activity Report And Data Analysis. It J E M 2017 (2), ePub
- Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions.Health Aff (Millwood). 2013 Dec;32(12):2149-56
- Komindr A, Baugh CW, Grossman SA, Bohan JS. Key operational characteristics in emergency department observation units: a comparative study between sites in the United States and Asia.Int J Emerg Med. 2014 Feb 5;7(1):6
- Gabele D, Bugais C, Laguna J.Medical Observation Units and Emergency Department Collaboration: Improving Patient Throughput.J Nurs Adm. 2016 Jul-Aug;46(7-8):360-5
- Epelde F, Iglesias-Lepine ML, Anarte L.[Economic crisis: cost and effectiveness of short stay hospital units].An Sist Sanit Navar. 2012 Sep-Dec;35(3):469-75
- Baugh CW, Venkatesh AK, Hilton JA, Samuel PA, Schuur JD, Bohan JS.Making greater use of dedicated hospital observation units for many short-stay patients could save $3.1 billion a year.Health Aff (Millwood). 2012 Oct;31(10):2314-23
- Deliberazione Regione Lazio 9/07/2004 n° 602 allegato 6 Sistema di remunerazione dell’emergenza.
- Deliberazione Regione Lazio 23/11/07 n° 946 Introduzione dell’Osservazione Breve Intensiva nel Lazio dal 10 gennaio 2008.
- Brillman J, Mathers-Dunbar L, Graff L, Joseph T, Leikin JB, Schultz C, et al. Management of observation units. American College of Emergency Physicians.Ann Emerg Med. 1995 Jun;25(6):823-30
Keywords
Thrombotic Thrombocytopenic Purpura, microangiopathic haemolytic anaemia, thrombotic events, multiorgan damage, plasmapheresis.
Introduction
Thrombotic Thrombocytopenic Purpura (TTP) is an acute syndrome characterized by the presence of microangiopathic haemolytic anaemia and thrombocytopenia.
The first case of TTP was described by Moschowitz in 1924 [1]. Recently, between 1982 and 2001, several authors [2-5] identified in the deficiency of the metalloproteinase ADAMTS13 the primary pathogenic cause. TTP is a rare disease [6-8]. it occurs between 30 and 50 years of age, more frequently in the female sex. In the absence of treatment, mortality exceeds 90%, while it is reduced to 10-20% after adequate plasma plasmapheresis or infusion therapy. However, half the deaths are attributable to complications associated with plasmapheresis and hospitalization (sepsis, haemorrhages, thrombosis, etc.) [9]
Case Report
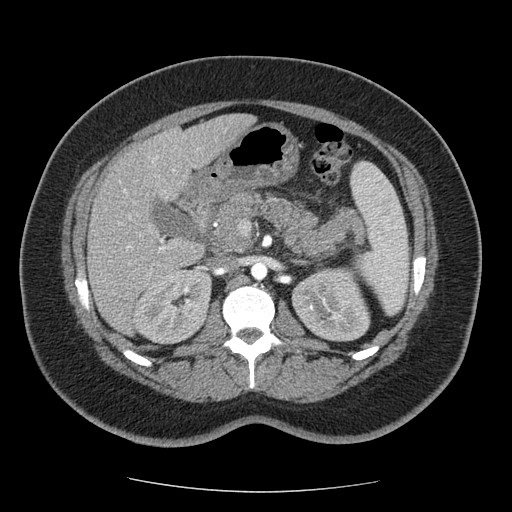
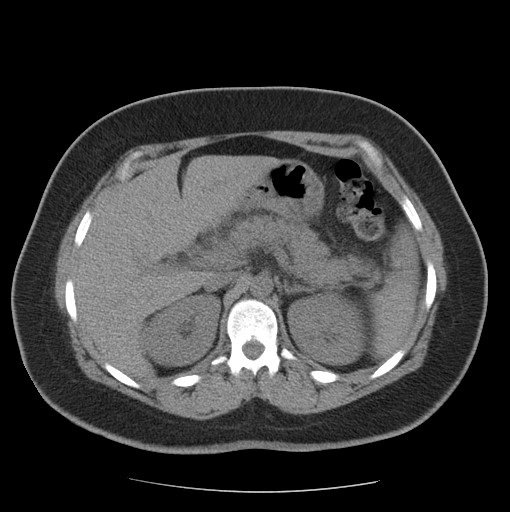
A 36-year-old italian woman came to our observation in the emergency department for abdominal pain, menometrorrhagia and vomiting. The patient had no significant past medical history. She had no significant clinical changes. She was hemodynamically stable.
Figure 1
Discussion
Conclusions
Our case report demonstrates the importance of the rapidity in setting the diagnosis and the treatment of PTT.
Therapy greatly reduces patient mortality. So far, the treatment performed by more physicians is not valid and nonconforming to the guidelines. Platelet transfusions are still administered by many increasing the risk of precipitating further thrombotic events. Their use is contra-indicated in TTP, unless there is life-threatening haemorrhage.
The symptomatology is often nonspecific and the diagnosis is posed with the presence of both microangiopathic hemolytic anaemia and thrombocytopenia. Multi-organ involvement, with the onset of acute pancreatitis, may be present.
However, symptoms demonstrating a multi-organ pathological involvement may be an expression of iatrogenic damage.
References
- Moschowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hitherto undescribed disease. Proc N Y Pathol Soc1924;24:21-4.
- Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factorVIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med 1982;307:1432-5.
- Furlan M, Robles R, La¨mmle B. Partial purification and characterizationof a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis.Blood 1996;87:4223-34.
- Tsai HM. Physiologic cleavage of von Willebrand factor by aplasma protease is dependent on its conformation and requires calcium ion. Blood 1996;87:4235-44.
- Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001;413:488-94.
- Scully M, Yarranton H, Liesner R, et al. (2008) Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. British Journal of Haematology,142, 819-826.
- Miller DP, Kaye JA, Shea K, et al. Incidence of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. Epidemiology 2004;15:208-15.
- Schech SD, Brinker A, Shatin D, Burgess M. New-onset and idiopathic thrombotic thrombocytopenic purpura: incidence, diagnostic validity, and potential risk factors. Am J Hematol 2006;81:657-63.
- George JN. The thrombotic thrombocytopenic purpura and hemolytic uremic syndromes: evaluation, management, and long-term outcomes experience of the Oklahoma TTP-HUS Registry, 1989-2007. Kidney Int Suppl 2009;112:S52-4
- Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med 2000;132:794-9.
- Miller RF, Scully M, Cohen H, et al. Thrombotic thrombocytopaenic purpura in HIV-infected patients. Int J STD AIDS 2005;16:538-42.
- George JN. Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med 2006;354:1927—35.
- Galbusera M, Noris M, Remuzzi G. Thrombotic thrombocytopenic purpura–then and now. Semin in Thromb and Hemost, 2006; 32, 81–89.
- McDonald V, Laffan M, Benjamin S, Bevan D, Machin S, Scully MA. Thrombotic thrombocytopenic purpura precipitated by acute pancreatitis: a report of seven cases from a regional UK TTP registry. Br J Haematol; 2009;144, 430–433.
- Burns ER, Lou Y, Pathak A. Morphologic diagnosis of thrombotic thrombocytopenic purpura. Am J Hematol 2004;75:18-21.
- George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood 2000;96:1223-9.
- Szczepiorkowski ZM, Bandarenko N, Kim HC, et al., American Society for Apheresis; Apheresis Applications Committee of the American Society for Apheresis. Guidelines on the use of therapeutic apheresis in clinical practice: evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher 2007;22:106-75.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991;325:393-7.
- Allford SL, Hunt BJ, Rose P, Machin SJ, Haemostasis and Thrombosis Task Force, British Committee for Standards in Haematology. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anaemias. Br J Haematol 2003;120:556-73.
- Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol 2007;136:451-61.
- Heidel F, Lipka DB, von Auer C, Huber C, Scharrer I, Hess G. Addition of rituximab to standard therapy improves response rate and progression-free survival in relapsed or refractory thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia. Thromb Haemost 2007;97:228-33.
- Foley SR, Webert K, Arnold DM, et al., Members of the Canada Apheresis Group (CAG). A Canadian phase II study evaluating the efficacy of rituximab in the management of patients with relapsed/refractory thrombotic thrombocytopenic purpura. Kidney Int Suppl 2009;112:S55-8.
- Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med 1982;307: 1432-5.