- Elisa Pizzolato
- Brief Report and Case Report
A “mushroom” grown up from the aorta
- 1/2017-Febbraio
- ISSN 2532-1285
Elisa Pizzolato, MD(1), Emanuele Bernardi, MD(1), Francesco Tosello, MD(1), Giulia Racca, MD(1), Letizia Barutta, MD(1), Bruno Tartaglino, MD(1), Giuseppe Lauria, MD(1), Attilio Allione, MD(1)
Emergency Department, S. Croce and Carle Hospital, Cuneo, Italy
Abstract
Acute abdominal pain is one of the most common symptoms of patients presenting in Emergency Department (ED). Abdominal pain etiology includes many diseases from mild to life-threatening causes. Aortitis is the inflammation of the aortic wall: it includes non-infectious and infectious vasculitis. In most cases of infectious aortitis there are preexisting vascular pathologies such as aneurysm sac or atherosclerotic plaque. Despite radiological and laboratory tests and aggressive medical and surgical therapies, mortality associated with infectious aortitis remains very high. We describe a case of an 82-year-old woman with abdominal periaortitis: it quickly evolved to an abdominal aortic pseudoaneurysm and unfortunately it complicated with patient’s death due to the pseudoaneurysm rupture.
Introduction
Acute abdominal pain is one of the most common symptoms of patients presenting at the Emergency Department (1): it’s defined as a non-traumatic pain experienced from less than 5 days and it represents the 10% of presentations in ED. The early diagnosis leads to an accurate management and results in less complications. Abdominal pain etiology includes variety of diseases ranging from mild and self-limiting to life-threatening diseases: they are classified in urgent and non-urgent causes. The urgent causes (appendicitis, acute diverticulitis, and bowel obstruction) need treatment within 24 hours from presentation, otherwise for non-urgent ones (nonspecific abdominal pain, NSAP, and gastro-intestinal diseases) immediate treatment is not required (2). The abdominal pain is a daily challenge for the emergency physician cause very often the first clinical presentation is unclear and non specific and evolve to more disease-specific symptoms over time. The diagnostic pathway starts with the clinical evaluation based on past and present medical history, physical evaluation and pain description (the acronym OPQRST helps physician to collect the necessary clinical data: O=pain onset, P=provocation or palliation, Q=pain quality, R=region and radiation, S=pain severity and T=time from the onset). A key aspect in the physical evaluation is the location cause it could be related with the pain cause. Laboratory test could be useful in the diagnosis process but they are not able to discriminate an urgent cause from an non-urgent one. Radiological imaging, such as plain radiography, ultrasound, and computed tomography (CT) has increased over the years: certainly, in many cases, it helps doctor during diagnostic pathway but we shouldn’t forget it also has important downsides (higher costs, increased risk of negative side effects such as contrast-induced nephropathy and ionizing radiation exposure) (3). Due to many underlying causes, in the area of many different specialties (gynecology, vascular and general surgery, gastroenterology, cardiology, urology, pneumology and toxicology) and despite the increased use of imaging modalities, alone or combined, the abdominal pain still remains a major diagnostic challenge (4).
Case Description
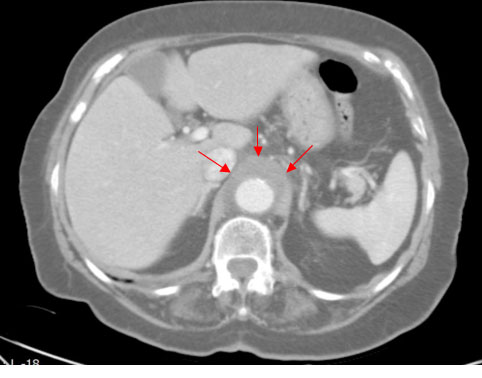
A 82-year-old woman was admitted to our ED because of chest and epigastric pain compared few days before. Her past medical history included coronary artery disease and polyarthrosis. Physical examination and electrocardiogram were normal, except for significantly different blood pressure values between arms raising suspect of a vascular complication. Five months before she admitted to our ED complaining of fever, abdominal pain and vomiting: the Computed Tomography (CT) scan showed an intestinal sub-occlusion and a periaortic tissue of unclear origin (Figure 1, arrows). At that time the patient was further examined by Positron Emission Tomography and rheumatologic evaluation with a final diagnosis of infectious periaortitis. She was treated with antibiotic therapy for 15 days (levofloxacin 500 mg q.d.) and she was suggested a radiological follow up with a CT control after 45 days: there weren’t at that time any indications for surgical treatment. She was discharged without fever and abdominal pain; laboratory tests showed reduction in systemic inflammatory markers.
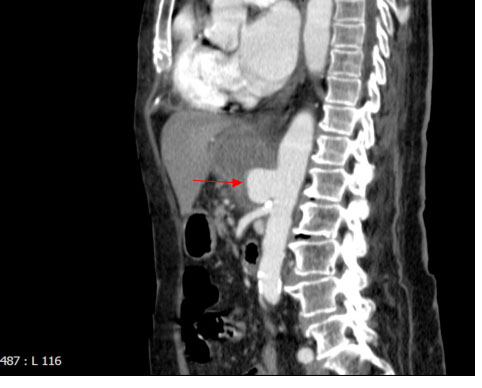
At the second ED admission, having regards to previous findings, she underwent an urgent abdominal CT scan which highlighted the evolution to an abdominal aortic pseudoaneurysm complicated by an abscess (Figure 2, arrow). She denied informed consent to surgical intervention and died four days later for a hemodynamic shock likely associated with pseudoaneurysm rupture.
Discussion
Aortitis is the general term used to defined inflammation of the aortic wall. The causes of aortitis are divided into non-infectious vasculitis (the most important are giant cell, or temporal arteritis, especially in older patients and Takayasu arteritis, in younger people) and, less commonly, infectious vasculitis (mostly due to Staphylococcus, Streptococcus, Pseudomonas, Salmonella and mycobacteria during last years and Treponema Pallidum and fungal infections in the past) (5). Suppurative aortitis generally results from endocarditic vegetations, from bacterial inoculation through penetrating trauma or may develop by direct extension of an adjacent infectious process (6). In most cases of infectious aortitis there are preexisting vascular pathologies such as aneurysm sac or atherosclerotic plaque (7). Atherosclerotic vascular diseases and chronic inflammatory periaortitis are among the most common etiologies of abdominal aortic aneurysms: in this setting, acute aortic syndrome occur in about 1-3% of subjects with 70-95% mortality (8). At each medical examination, a through radiological exam in combination with inflammation biomarkers should be perform in order to early identify clinical complications (acute aortic syndrome). Surgical indications for aortic aneurysms due to inflammation disorders (such as infectious and non-infectious aortitis) are the same to those in non-inflammatory disorders. Physicians should remember that patient must be in clinical inflammation remission before surgical repair, cause the risk of a graft failure is much greater in patient with active local inflammation (5). The ESC guidelines task force suggest rapid intravenous antibiotics administration with broad antimicrobial coverage of the most likely pathological organisms. Antibiotics therapy should be initiated as soon as infectious aortitis diagnosis is suspected without waiting for blood culture results. Although neither international guidelines nor clinical studies established the optimal duration of antimicrobical therapy, a medical treatment of 6-16 weeks after surgical intervention or clearance of blood cultures and more of 16 weeks for immunocompromised patients is generally recommended (7,9). Despite aggressive medical and surgical therapies, mortality associated with infectious aortitis remains high largely owing to aortic rupture (7): Hsu et colleagues showed, in a 46 patients clinical study, the 3% in 30-days-mortality in patients who underwent surgical therapy and 45% in the group of medical therapy (9) . For this reason, in the work up of abdominal pain, particularly in subjects carrying previous findings associated with inflammatory periaortitis, acute abdominal aortic syndrome should be excluded as soon as possible. Due to the increasing number of aged patients with atherosclerosis the management of infected aneurysm is thought to be becoming more important.
Teaching Point
The case we studied showed a very quick evolution from the first diagnosis of infectious aortitis to the time of aneurysm formation. Furthermore, the infectious aneurysm quickly developed to the lethal rupture.
In presence of aortic aneurism, the follow up strategy normally consists in monitoring the aneurism every year: if the aneurism diameter grows more than 1 cm the surgical or endovascular treatments are indicated. Surgical and endovascular treatments are also indicated if aneurysm diameter is more that 5-6 cm or if the patient is symptomatic (10).
References
- Kamin RA, Nowicki TA, Courtney DS, et al. Pearls and pitfalls in the emergency department evaluation of abdominal pain. Emerg Med Clin North Am 2003; 21: 61–72.
- Lameris W, van Randen A, van Es HW, et al. Imaging strategies for detection of urgent conditions in patients with acute abdominal pain: diagnostic accuracy study. BMJ 2009; 338:b2431.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35 year retrospective. Am J Emerg Med 2011; 29: 711–716.
- Gans SL, Pols MA, Stoker J et al. Guideline for the Diagnostic Pathway in Patients with Acute Abdominal Pain. Dig Surg 2015;32:23–31.
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. European Heart Journal (2014) 35, 2873–2926.
- Ladich E, Yahagi K, Romero ME, et al. Vascular diseases: aortitis, aortic aneurysms, and vascular calcification. Cardiovascular Pathology 25 (2016) 432–441.
- Gornik HL, Creager MA. Aortitis. Circulation 2008;117:3039-3051. doi: 10.1161/CIRCULATIONAHA.107.760686.
- Schirmer JH, Both M, Moosig F. Chronic periaortitis. Internist (Berl) 2013;54: 1419-20,1422,1424-6. doi: 10.1007/s00108-013-3297-5.
- Hsu RB, Chen RJ, Shen SW, et al. Infected aortic aneurysms: Clinical outcome and risk factor analysis. J Vasc Surg 2004;40:305.
- Di Carlo C, Anzidei M , Napoli A. Imaging cardiovascolare TC e RM: Dalla tecnica all’interpretazione clinica. Springer-Verlag Italia 2012. doi 10.1007/978-88-470-2604-9.

